UTK Environmental Health & Safety Program LS-BIO-004
Effective Date: 11/17/2023
Revision Date: 11/17/2023
Purpose
Centrifugation may present two serious hazards: mechanical failure and dispersion of aerosols. This document provides guidance for the safe operation of centrifuges to minimize the risk of exposure to infectious/hazardous materials; however, it is the responsibility of the owners and operators to understand and follow proper use of their specific centrifuge and laboratory equipment.
Scope and Applicability
The information and practices in this document apply to the use of centrifuges at UTK, UTIA, and GSM.
Definitions and Abbreviations
Definitions
Centrifuge: A machine with a rapidly rotating container that applies centrifugal force to its contents, typically to separate fluids of different densities or liquids from solids. Examples are in Figure 1.

Figure 1: Examples of different types of centrifuges.
Rotor: The object that houses sample tubes and is spun around a fixed axis, generating centrifugal force. The two most common types of rotors are the fixed-angle rotor and the swinging bucket rotor. The fixed-angle rotor, see Figure 2, is a solid rotor with holes to hold centrifuge tubes, usually set at a 45° angle. The swinging bucket rotor, see Figure 3, consists of rigid arms from which a bucket with various inserts can be hung. The buckets freely swing to a horizontal orientation when the centrifuge is in operation.

Figure 2: Labeled centrifuge with fixed angle rotor
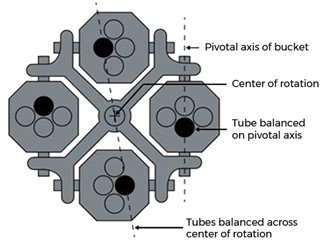
Figure 3: Diagram of swinging bucket rotor
Abbreviations
BSL: Biological safety level
GSM: Graduate School of Medicine
PI: Principal Investigator
PPE: Personal Protective Equipment
SOP: Standard Operating Procedure
UTK: University of Tennessee Knoxville main campus
UTIA: University of Tennessee Institute of Agriculture
Introduction
The centrifuge is a commonly used tool in laboratory research, but they can also be dangerous when not used and maintained properly. It uses centrifugal force to separate substances in liquid or solid media according to particle size and density differences. This centrifugal force is created by rapidly spinning the rotor around the axis. Centrifuges can undergo thousands of rotations per minute and create relative centrifugal forces several thousand times that of gravity. Mechanical failure and dispersion of aerosols can present hazards when using a centrifuge. Therefore, training on how to use the centrifuge properly and safely is essential for all new employees as part of Lab-Specific Training.
Before Centrifugation
- Train each operator on proper safety and operating procedures, including a review of the user manual.
- Use only rotors compatible with the centrifuge. Check the expiration date for ultracentrifuge rotors.
- Check tubes, bottles, and rotors for damage and deformities before each use.
- Make sure that any surfaces where liquid or solid contaminants could accumulate (such as the rotor, tubes, spindle/axle) are dry and clean.
- Examine O-rings and replace if worn, cracked, or missing.
- Never overfill centrifuge tubes (don’t exceed ¾ full).
- Always cap tubes before centrifugation.
- Tubes with gasketed lids can provide additional containment.
- Check that the rotor is seated and secured on the drive correctly, close and secure the lid of the centrifuge.
- When using swinging bucket rotors, make sure that all buckets are hooked correctly and move freely.
- Always balance buckets, tubes, and rotors properly, see Figure 4. Pay attention to volumes in tubes, weight of swinging bucket inserts, etc.
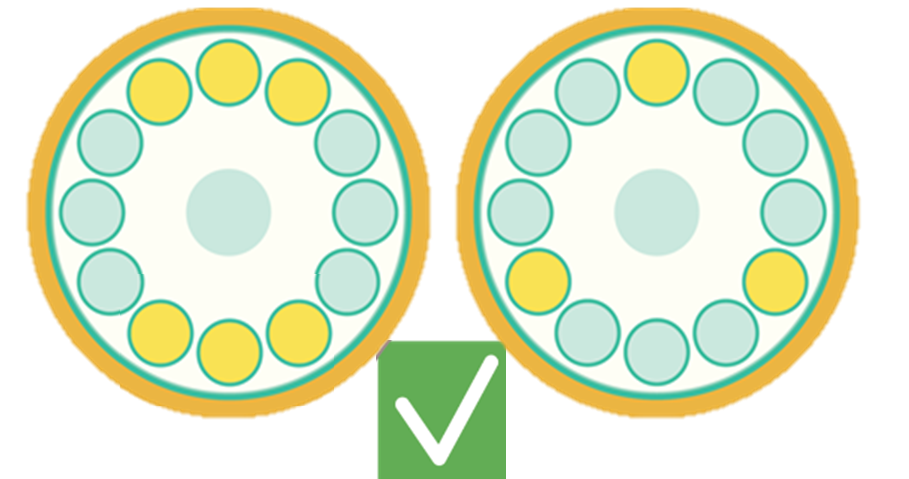

Figure 4: Examples of proper and improper balancing
During centrifugation
- Keep the lid closed at all times during operation. Never open a centrifuge until the rotor has stopped.
- Do not exceed safe rotor speed. This will differ among individual centrifuges and rotors. It is your responsibility to know the range of safe operating speeds for your equipment (see operator’s manual for details).
- The operator should not leave the centrifuge until full operating speed is attained and the machine appears to be running safely without vibration.
- Stop the centrifuge immediately if an unusual condition (noise or vibration) begins and check load balances.
After centrifugation
- Allow the centrifuge to come to a complete stop before opening.
- Wear appropriate PPE including gloves to remove rotor and samples.
- Check inside the centrifuge for possible spills and leaks; clean and disinfect the centrifuge and rotor thoroughly if necessary.
- Wash hands after removing gloves.
Centrifuging Infectious Materials or Human Samples
Follow the safety procedures above, plus:
- Place a biohazard label on the centrifuge.
- Always wear gloves when handling tubes or rotors.
- Ensure that the tube material is compatible with the sample to reduce the risk of tube malfunction/failure.
- Avoid the use of celluloid tubes with biohazards. If celluloid tubes must be used, an appropriate disinfectant must be used to decontaminate them.
- Always use sealed safety cups, safety buckets, or sealed rotors with O-ring if available, see Figure 5. (O-ring indicated by the blue arrow). If engineering controls are not available, work practice controls must be in place when centrifuging materials requiring BSL-2 containment. Work practice controls are low speeds with low volumes and/or settling times (5-10 minutes).

5a

5b
Figure 5: Example of swing bucket rotor with safety cups (A); and fixed rotor with engineered with bioseal technology (B) (O-ring indicated by blue arrow).
- Fill centrifuge tubes, load them into rotors, remove from rotors, and open tubes within a biological safety cabinet if available.
- Wipe exterior of tubes or bottles with disinfectant prior to loading into rotor or bucket. Seal rotor or bucket, remove outer gloves, and transport to the centrifuge.
- If possible, wait approximately 5-10 minutes after the run to allow aerosols to settle before opening the centrifuge. If a settling time is not feasible, additional safety measures (e.g. enhanced PPE) may be required if determined by risk assessment.
- Routinely check for possible spills or leaks. For spills of infectious materials, see centrifuge emergency procedures below.
- Decontaminate centrifuge interior, safety cups or buckets, and rotors if tube leakage or breakage occurs.
- Include centrifugation procedure and decontamination plan in lab SOPs.
Emergency Situations
The following events are considered an emergency:
- Spill in the centrifuge
- Centrifuge malfunctions
- Rotor failure
- Tube breakage
Emergency Procedures
For emergencies resulting in the release of infectious aerosols or other types of hazardous materials:
- Evacuate the lab if necessary.
- Notify others.
- Seek medical attention as necessary.
- If possible, turn the centrifuge off immediately and keep centrifuge lid closed.
- Close the lab door.
- Post a sign at the lab door describing the agent and emergency.
- Report incidents to PI or lab supervisor. Call EHS at 865- 974-5084 for assistance, especially when hazardous chemicals, infectious agents, or radiological materials are involved.
- If spill cleanup is advised by EHS, the operator should wear proper PPE, remove debris, clean, and decontaminate/disinfect centrifuge interior, rotors, safety cups or buckets following the manufacturer’s instructions and your lab’s spill response plans.
- Place any contaminated protective clothing, gloves, and all cleanup materials in a biohazard bag or hazardous waste bag.
- Wash hands and any exposed skin surfaces with soap and water.
Procedures for other described situations (rotor failure/centrifuge malfunction)?
References
UT System Policy SA0100
UT System Policy SA0450
https://www.fishersci.se/se/en/scientific-products/centrifuge-guide/centrifugation-theory.html#tab4