UTK Environmental Health & Safety Guide LS-018
This document summarized the requirements of health and safe research laboratory
Effective Date: 07/29/2022
Revision Date: 07/29/2022
Purpose
It is essential for those who perform tasks involving sharp devices (sharps) to do so in a manner that minimizes the potential for injuries and exposures to hazardous agents This document provides a framework for the safe and prudent use, management, and disposal of sharps devices, particularly those associated with hazardous agents (biological, chemical, or radiological).
Scope and Applicability
The safety practices outlined in this document apply to sharps use and management in the research, teaching, and diagnostic testing in UTK and GSM laboratories, shops and maker spaces. Safety practices covering biohazardous sharps extend to the same settings at UTIA.
Abbreviations and Definitions
Abbreviations
UTK: University of Tennessee, Knoxville
UTIA: University of Tennessee Institute of Agriculture
FDA: Food and Drug Administration
GSM: Graduate School of Medicine
TDEC: Tennessee Department of Environment and Conservation
OSHA: Occupational Safety and Health Administration
Definitions
Sharps – Sharps are a broad category of devices that are sharp enough to puncture or lacerate the skin without excessive applied pressure or force, examples include but are not limited to:
- Hypodermic or suture needles
- Scalpel blades
- Knives (or similar objects with exposed cutting edges)
- Pasteur pipettes
- Capillary tubes
- Microscope slides and coverslips
- Broken or unpolished glass
Safety Considerations
Eliminate the use of sharps (including glass) whenever possible.
Carefully review procedures involving sharps. Non-sharps alternatives are often available, inexpensive, easy to use, and eliminate (or greatly reduce) the risk of injury and/or exposure.
Glass septum vials, capillary tubes, Pasteur pipettes and slides/coverslips often have unpolished (sharp) edges and/or are easily broken, creating sharp edges. Consider non-glass alternatives that eliminate the need to use these breakable devices. If you can’t eliminate their use, consider options that have shatter-proof features (i.e. Teflon coating).
Select a safety-engineered sharps device that will accomplish the same result while also lowering your risk of injury.
If sharps are required for procedures, it is prudent and sometimes required (see below) to use a safety-engineered sharps device to lower the risk of injury and exposure. Examples include, but are not limited to:
- Self-sheathing needles/syringes
- Hypodermic syringes with retractable technology safety features
- Phlebotomy needles with self-blunting safety features
- Retracting lancets with safety features
- Disposable scalpels with shields or similar safety features
- Blunt-ended tissue scissors.
The Occupational Health Administration (OSHA) Bloodborne Pathogens Standard requires the use of safety-engineered sharps when used in conjunction with human blood, body fluids, or other potentially infectious materials (e.g. unfixed organs and tissues), particularly in human healthcare settings. While this provision does not extend to some academic and research settings, the use of such devices is strongly recommended whenever possible. Please contact the UTK EHS-Biosafety at (865) 974-5084 or utbiosafety@utk.edu for assistance regarding sharps with safety features and/or further information on the OSHA Bloodborne Pathogens Standard. Additional information is also available through the International Sharps Injury Prevention Society.
Prepare written procedures.
Develop written procedures for specific instances of sharps use that describes:
- Tools and instruments that are needed to perform the procedure safely.
- Personal protective equipment.
- Steps to perform the procedure safely.
- Steps for responding to an accident, injury, or exposure.
Participate in training on proper techniques before using sharp devices.
Improper use of sharps and poor technique can increase your risk of sustaining an injury or exposure. Ensure that you have been properly trained by senior personnel on the sharp device, its use and limitations, and respective safe handling techniques. A dry run (i.e. without other hazards present) to ensure sharps handling proficiency is strongly encouraged.
Use needles in the appropriate and safe manner.
- When using needles and syringes, use only those with locking systems (e.g. Luer lock) or a fixed/integrated feature. Slip-tip needles/syringes should be avoided.
- Do not bend, break, or shear needles.
- Point the needle away from yourself.
- Keep visual contact with the needle while uncapped.
- Place a sharps container in the vicinity of the work and place the uncapped needle directly in the sharps container immediately after use. Never place an uncapped needle unattended on a workbench.
- Do not recap needles unless recapping is required by the procedure or not recapping creates a higher risk of injury than recapping.
- If the needle must be recapped, use the one-handed scoop technique as illustrated in Figure 1. If the one-handed scoop technique is not feasible, use a needle holder, recapping device, or other method that does not put a body part in the line of fire.
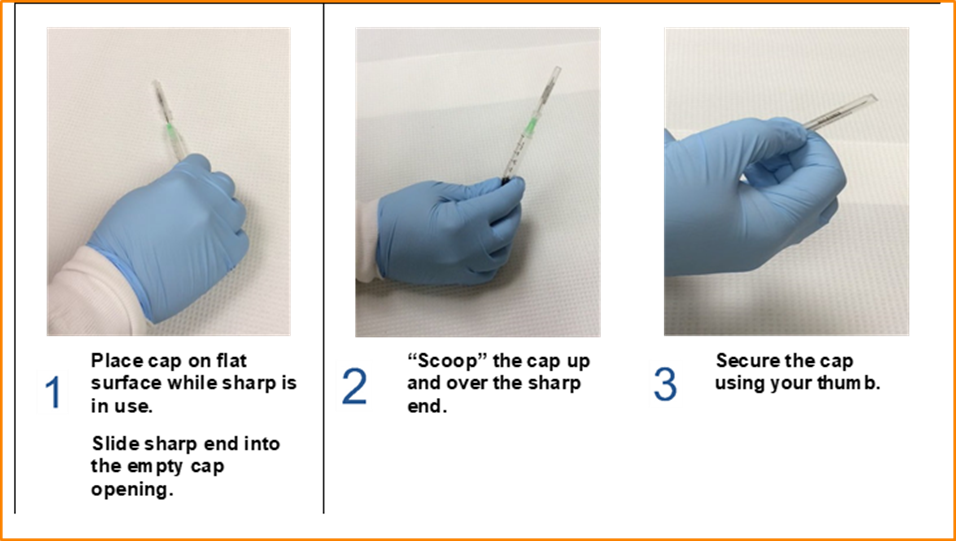
Fig. 1 One-handed scoop technique
- If needle removal is necessary, do not remove an exposed needle with your hands. Recap the needle (as described above) prior to removal if possible. Otherwise, use a tool that is engineered specifically for needle removal. If an engineered tool is not available, use a tool such as pliers or hemostats to remove the needle.
- Do not use syringes with needles attached as specimen containers if other alternatives are available. If other alternatives are not available, recap the needle (as described above) and place syringe in leak-proof secondary container with a secure closure for transport.
Use scalpels/blades in the appropriate and safe manner.
- Before using a disposable blade, stage a sharps container within arm’s reach so that it can be immediately disposed of after use.
- Do not use blades without a handle.
- Use disposable safety scalpels with fixed blades whenever possible. These devices eliminate the need to remove a blade, which requires significant skill to perform safety.
- If you must use a reusable scalpel, consider using blades with engineered safety features that allow for enclosure of the blade before blade removal. If safety engineered blades are not an option, forceps or other mechanical equipment must be used to remove the blade.
- Do not saw with a scalpel or put excessive force on it. These actions can cause the blade to snap creating an aerosol and flying debris hazard as well as a sharps exposure hazard. Use knives or purpose-designed blades for tasks that require greater cutting action.
- Do not leave blades out in the lab environment after use (i.e., left on the lab counter), regardless of what they have been used for.
Eliminate or modify two-handed methods when using sharps.
Recapping needles, passing sharp devices (i.e., scalpels) from one hand to another, or handing sharp devices to another person are common examples of two-handed techniques that can lead to injuries. Eliminate these techniques or modify the technique to eliminate the risk to the non-dominant hand or other body parts that may be placed in the line of fire. Examples include, but are not limited to:
- Recapping needles using the one-handed scoop technique.
- Adopting a system to prohibit personnel from grasping the device at the same time if a sharp device must be passed between personnel. An example of how this can be achieved is for the person who’s in control of the sharp to place it on a suitable surface in a manner that the next user can easily grasp the handle instead of the sharp end.
- When conducting tissue collection, have one person in control of the sharp device. If manual bracing is necessary, the bracing hand must always be behind the sharp device/cutting instrument. Cut away from the bracing hand, never towards! Assisting personnel should have their hands as far away from the cutting area as feasible and pay attention to the person handling the sharp device. Additionally, the use of cut-resistant gloves (especially on the non-dominant hand) is recommended for procedures that present a likelihood of exposure to a cutting device.
- When cleaning and processing reusable sharps, use cleaning tools that limit the potential for contact between your hands and the sharps surfaces.
- When removing a scalpel blade from a reusable scalpel handle, use a mechanical device such as a hemostat to remove the blade. Deposit the blade directly into a sharps container using the mechanical device. Alternatively, use automatic scalpel blade removers, which are available from most scientific supply companies.
Additional sharps safety precautions.
- Do not leave sharps unattended when preparing them for use or after they have been used.
- Do not leave sharp devices in your lab coat, coveralls, or scrub pockets!
- For disposable sharps, have a sharps container near the work for disposal of sharps immediately after use.
- Do not bend, break, or put excessive force on a sharp device. These actions increase your risk of sustaining a sharps injury.
- For transporting reusable sharps (i.e., knives, scissors), have a storage container that will enclose the sharp end (i.e., a bucket or enclosed tray) readily available at the point of use.
- For storing reusable sharps, use a method that allows visibility and safe selection. Figure 2 illustrates a magnetic strip that can be used for safe sharps storage (attention must be paid to the orientation of the sharp ends and edges).

Figure 2. Magnetic strip for safe storage of sharp tools and devices.
Sharps Waste Disposal Management
Uncontaminated Broken Lab Glassware
Uncontaminated broken lab glassware may cause cuts, punctures, or other physical injuries. To dispose, collect in a puncture-proof container (e.g., plastic container or broken glass box), close/tape the container closed, label as broken glass, and take to the dumpster for disposal. For questions, contact EHS at ehs_labsafety@utk.edu or (865) 974-5084.
Uncontaminated sharps
Uncontaminated sharps may cause cuts, punctures, or other physical injuries. Collect in a rigid, puncture-proof container (non-glass) that can be securely closed. Clearly label the containers as uncontaminated sharps. To dispose, place closed containers in the red bins provided by the UTK medical waste contractor or submit to EHS during scheduled hazardous waste pickups.
Sharps with biological/infectious contamination
Biologically contaminated sharps used in clinical patient care (human and animal) and/or medical, research, or industrial laboratories are regulated as medical waste by the Tennessee Department of Environment & Conservation (TDEC). Examples include, but are not limited to: hypodermic needles with/without attached syringes, contaminated syringe barrels, Pasteur pipettes, scalpel blades, blood vials, needles with attached tubing, and contaminated broken or unpolished glassware (e.g. used slides and coverslips). For disposal:
- Use an FDA-approved sharps container that is puncture- and leak-proof on the sides and bottom, has a means of permanent closure, and bears the biohazard symbol. Makeshift containers such as milk jugs, fluids bottles, bleach bottles, etc., are not appropriate sharps containers.
- Ensure the lid is properly installed before putting the container into use.
- Use a container that is the appropriate size and configuration for the sharps waste being generated. Items should fall freely into the container without excessive manipulation. Sharps should never be bent, broken, or otherwise forced into a sharps container. Sharps containers with passive drop mechanisms should be used whenever possible.
- Do not fill sharps containers past the ‘FULL’ mark (see Figure 3); otherwise, do not fill beyond ¾ of the container’s capacity. If items do not freely fall, the container is too full and/or incorrectly sized for the disposed sharps.

Figure 3. Fill capacity indicator on a sharps container.
- Avoid using sharps containers for non-sharps waste such as gloves, plastic tubes, gauze, or paper towels. These items take up space in the container and can hinder sharp items from falling into the container effectively.
- Do not shake sharps containers to try to make more room in them. This creates aerosols and can cause items to come out of the containers.
- NEVER retrieve an item from the container with your hand.
- Close the lid for transport or storage purposes. Permanently close the lid prior and wipe the outside of the container with disinfectant prior to disposal.
- Place permanently closed containers in the red bins provided by the UTK medical waste contractor or submit to EHS during scheduled hazardous waste pickups
NOTE: While there are some regulatory exemptions to waste regulations for household and farm operations, these exemptions do not extend to university operations. Therefore, biologically contaminated sharps waste must be collected in an appropriate sharps container as described above. Alternative collection devices for remote areas (e.g., Research and Education Centers) that may have infrequent access to transportation and disposal, must be approved by EHS-Biosafety and issued specialized container labels as shown in Figure 4.

Figure 4A Examples of an acceptable alternative sharps container

Figure 4B. Examples of a sharps container required label
Request for approval of an alternative collection device can be made by filling out this form: Alternative Collection Device.
Sharps with chemical contamination
Collect in an opaque, puncture-proof container that can be closed/sealed. The sharps container should not be red/orange or bear the biohazard label as the EHS chemical waste contractor will not accept sharps waste containers that resemble biohazardous waste. Label with the UTK hazardous waste label/description per EHS hazardous waste procedures. Submit full containers to EHS during scheduled hazardous waste pickups. Contact EHS for additional guidance on acceptable collection containers and/or disposal procedures.
Sharps contaminated with trace chemotherapeutic agents must be collected in yellow containers, which are to be packaged in designated cardboard boxes (not large red bins) for incineration by the UTK medical waste contractor; see Figure 5.

Figure 5A. Acceptable container of sharps contaminated with chemotherapy residues

Figure 5B. Contractor-provided cardboard box for incineration
Sharps with radiological contamination
Collect in an opaque, puncture-proof container that can be closed/sealed. The sharps container should not be red/orange or bear the biohazard label. Submit full containers to Radiation Safety with the appropriate hazardous label/description per Radiation Safety procedures. Contact (865) 974-5084 or radiationsafety@utk.edu for additional guidance on acceptable collection containers and/or disposal procedures.
References
Regulations and Standards
29 CFR 1910.1030 OSHA Bloodborne Pathogens Standard
TDEC Rule 0400-11-01-.04(2)(k)(4)
UT Policy
UT System Safety Policy SA0100 – Safety and Environmental Health Program
UT System Safety Policy SA0450 – Biological & Select Agents
UTK Programs, Procedures, Plans, and Guides
UTK Biological Safety Program – LS-BIO-001
UTK Hazardous Waste Management Plan – EC-001
UTK Laboratory Health & Safety Program – LS-001
Disclaimer
The information provided in these guidelines is designed for educational use only and is not a substitute for specific training or experience.
The University of Tennessee Knoxville and the authors of these guidelines assume no liability for any individual’s use of or reliance upon any material contained or referenced herein. The material contained in these guidelines may not be the most current.
This material may be freely distributed for nonprofit educational use. However, if included in publications, written or electronic, attributions must be made to the author. Commercial use of this material is prohibited without express written permission from the author.